Transform your research compliance management and admin operations with our specialized solutions.

Optimize the IRB compliance process to minimize investigator, coordinator, and committee effort while ensuring stringent approval protocols and risk mitigation.
Explore More →
Facilitate the review and monitoring of all animal research protocols, ensuring full compliance with IACUC regulations for both USDA and non-USDA species.
Explore More →
Protect personnel, human subjects, and society from biohazards by efficiently reviewing research protocols involving carcinogens, infectious agents, and allergens.
Explore More →
Streamline the approval and documentation process for stem cell research, ensuring consistent oversight and compliance with SCRO guidelines.
Explore More →
Simplify CSC compliance by automating protocol submissions, ensuring safer use of hazardous chemicals and stronger oversight in research environments.
Explore More →
Simplify CS compliance by guiding research teams through complex regulations governing the use of drugs and controlled substances.
Explore More →
Ensure safe and compliant use of radioactive materials in research through structured RSC protocol review and enforcement.
Explore More →
Facilitate regular inspections of approved protocols and swiftly implement corrective actions to ensure continuous compliance with established safety and research standards.
Explore More →








Streamline grant administration with an automated system for tracking applications, approvals, and compliance. This saves time, reduces errors, and ensures funds are allocated correctly and efficiently.
Explore More →
Simplify conflict of interest tracking with a digital solution that automates disclosure management, approval workflows, and audits. This reduces risks and ensures full transparency for all stakeholders.
Explore More →
Enhance post-award oversight by automating tracking, reporting, and compliance monitoring after the grant is awarded. This ensures funds are used correctly and compliance is maintained throughout the project.
Explore More →



Make animal research compliance easier by automating protocol reviews and tracking health records. This reduces administrative work while ensuring ethical and regulatory standards are met.
Explore More →
Optimize lab animal resource management with a streamlined software solution that tracks inventory, usage, and compliance. This enhances efficiency, reduces errors, and ensures better resource allocation.
Explore More →
Strengthen animal health and safety oversight with a centralized system for monitoring animal welfare, safety protocols, and compliance. This ensures humane treatment and minimizes research risks.
Explore More →
Facilitate regular inspections of approved protocols and swiftly implement corrective actions to ensure continuous compliance with established safety and research standards.
Explore More →


Make animal research compliance easier by automating protocol reviews and tracking health records. This reduces administrative work while ensuring ethical and regulatory standards are met.
Explore More →
Facilitate the review and monitoring of all animal research protocols, ensuring full compliance with IACUC regulations for both USDA and non-USDA species.
Explore More →
Unify and streamline your data management with our comprehensive data infrastructure services, ensuring your data is accessible, secure, and consistent. Integrate disparate data sources into a single platform to improve data integrity, governance, and facilitate smarter business decisions.
Explore More →
Simplify your hosting experience with our reliable and scalable hosting services, offering robust security, seamless performance, and compliance. By trusting us with your digital infrastructure, you can focus on innovation and growth while we ensure your applications run optimally and securely.
Explore More →

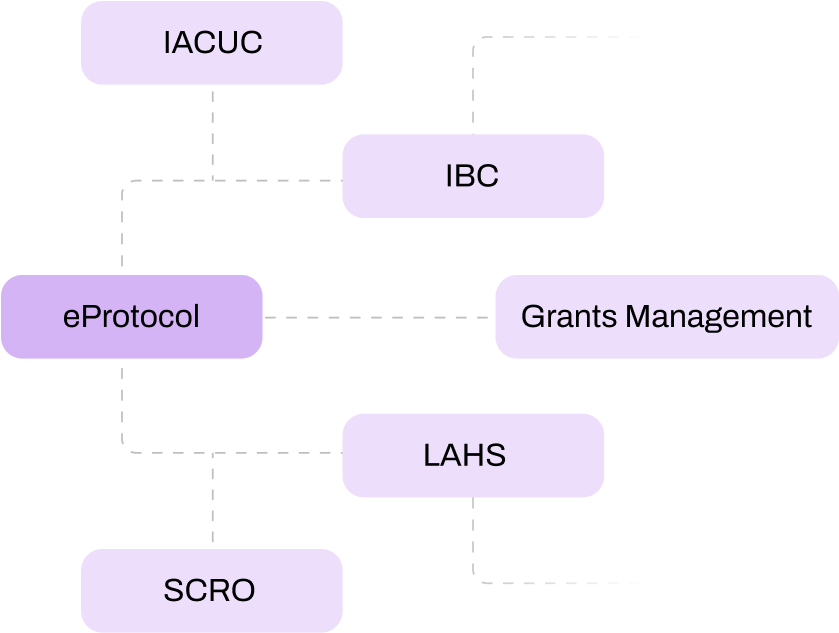
We understand university timelines, academic calendars, and the reality that you cannot shut down research operations for a software implementation.

Start with one committee or department, prove the system works, then expand. No "complex and long" implementations that disrupt ongoing research during the academic year.

Reduce trial delays, streamline workflows, and speed up product development from clinical trials to market launch.

Reduce trial delays, streamline workflows, and speed up product development from clinical trials to market launch.
Explore our upcoming events and see what’s next in compliance and digital solutions.