Human Subjects Research:
Individuals who are the subjects of research will be asked to contribute their time and assume risk to advance the research enterprise, which benefits society at large. U.S. federal regulations governing the protection of human subjects in research have been in existence for more than three decades. The Department of Health, Education, and Welfare (HWE) first published regulations for the protection of human subjects in 1974. The Department of Health and Human Services (HHS), formally known as HWE. There have been many revisions to 45CFR 46.
Federal Regulations for the protection of human subjects include:
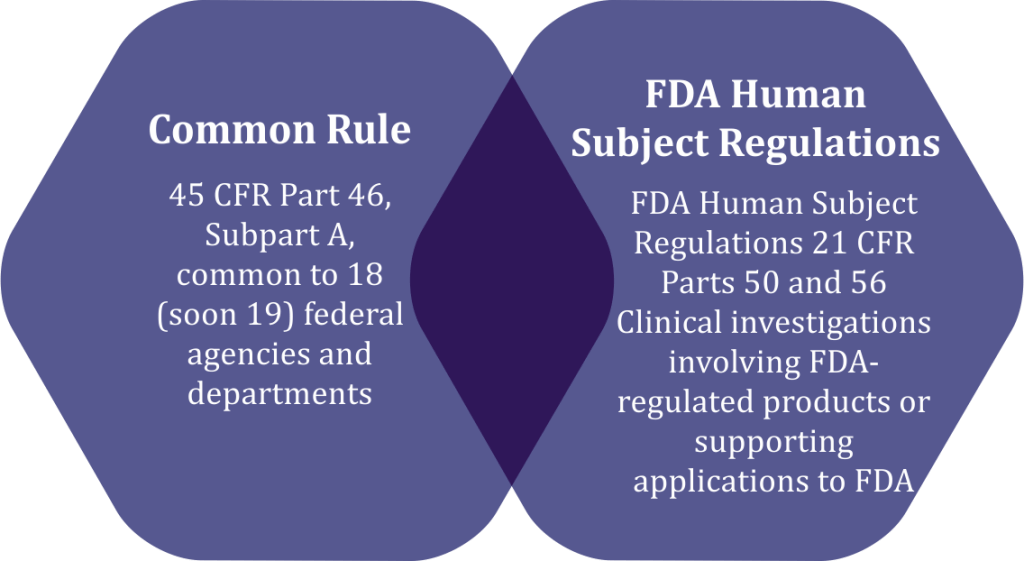
1. HHS Regulations are categorized under 45 CFR 46 and subdivided into 4 parts.
Common Rule – The current U.S. system of protection for human research subjects is heavily influenced by the Belmont Report, written in 1979 by the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Federal Policy for the Protection of Human Subjects or the “Common Rule” was published in 1991 and codified in separate regulations by 15 Federal departments and agencies
2. Food and Drug Administration (FDA) Regulations can be found under 21 CFR parts 50 and 56.
These regulations are applicable to regulators, research agencies, investigators and sponsors and the regulations will be applied according to the study. Further, there are certain similarities in the existing Common Rule and the FDA regulations such as IRBreview and informed consent. While each of these were built upon the other during development, there are some differences that must be taken into consideration by all stakeholders. Examples include qualifications for exemptions and the requirements for waivers of consent.
The Common Rule**:
Relevant Regulatory Background & History:
The “Common Rule,” also known as the Federal Policy for the Protection of Human Subjects, is the common ethical standard for publicly funded research in the United States.
The Rationale behind changing the Common Rule:
- The changing nature of research
- Public Comments, Export Advice and Stakeholders dialogue
- Signatories to Common Rule
The Revised Common Rule:
Since the Common Rule was promulgated, the volume and landscape of research involving human subjects have changed considerably. Research with human subjects has grown in scale and become more diverse. Examples of developments include: an expansion in the number and types of clinical trials, as well as an increase in the number of observational studies and cohort studies; a diversification of the types of social and behavioral research being used in human subjects research; increased use of sophisticated analytic techniques to study human biospecimens; and the growing use of electronic health data and other digital records to enable very large datasets to be rapidly analysed and combined in novel ways. Yet these developments have not been accompanied by changes in the human subjects research oversight system, which have remained largely unaltered over the past three decades.
As a result of the changes in the health care industry that were witnessed between 2009 and 2011, the Office of the Secretary of HHS, worked in conjunction with the Executive Office of the President’s Office of Science and Technology Policy (OSTP) to identify needs for updating the then regulations to “modernize” and a make the regulations “more effective.”
A lengthy rule making process was undertaken to:
- Modernize regulations to reflect significant changes in volume and landscape
- Facilitate research including research involving genetics and to reduce burden, delay and ambiguity
- Streamline IRB review
- Address the ethical debate regarding secondary research with non-identifiable biospecimens
The Revised Common Rule guidelines were issued on Jan 19, 2017 (82 F.R. 7149) and were to be made effective from Jan 19, 2018 with compliance date on Single IRB-of-Record (sIRB) requirement of Jan 20, 2020. However, the implementation of the Revised Common Rule was delayed and to be implemented on Jan 21, 2019.
If your institution decides to take advantage of any one of the three “burden-reducing provisions,” on or after July 19, 2018, the study must be in full compliance with all the Revised Common Rule regulations by Jan 21, 2019.
Three “burden-reducing provisions”:
- Usage of the revised definition of “research,” which now removes four of the categories as research
- The allowance for no annual continuing review of certain categories of research
- The elimination of the requirement that institutional review boards (IRBs) review grant applications or other funding proposals related to the research
Why the industry should pay attention to the “Revised Common Rule?”
Though the Revised Common Rule is applicable only for Federally Funded Research; still from the industry’s perspective it requires attention because of what it holds for the industry
- Direct Impact: It will lead to change in FDA regulations eventually as 21st Century Cures Act mandates harmonization between Common Rule and FDA Human Subjects Regulations.
- Indirect Impact: The Common Rule is and will continue to be the default framework under which the industry’s institutional collaborators conduct human subjects research paving way to changes in institutional processes, policies and forms.
Key provisions in the Revised Common Rule & the potential implications for the industry
- Continuing Review: Under the Revised Common Rule it is no longer required for some research that has progressed to the point that it involves only one or both of the following, which are part of the IRB-approved study: (1) Data analysis, including analysis of identifiable private information or identifiable biospecimens, or (2) Accessing follow-up clinical data from procedures that subjects would undergo as part of clinical care. The IRB will notify researchers upon initial approval about any post-approval review requirements.
- Exemptions: The categories of research that are exempt from IRB review have been expanded under the Revised Common Rule and furthermore some existing categories have been clarified. Some exemptions may require “Limited IRB review”, while others may qualify for “self-determination.” Under the revised rule, researchers will still submit all human participant research to the IRB to determine any exemptions.
- Informed Consent: A new “Key Elements” section and rearranged content introduced in the Revised Common Rule aims to facilitate a potential research subject’s decision to participate or not. A new option for broad consent may be available in the future.
- Single IRB-of-Record (sIRB): Most federally funded collaborative research projects located in the US that require IRB oversight will be required to use a single IRB (commercial, academic, or hospital-based) starting January 2020.
**For your knowledge the Common Rule is now referred to as the “Pre-2018 Requirements” and the Revised Common Rule is referred to as the “2018 Requirements.” If your institution decides to adapt any of the “three burden-reducing provisions,” it may be easier to use the new reference language.
More information: Link