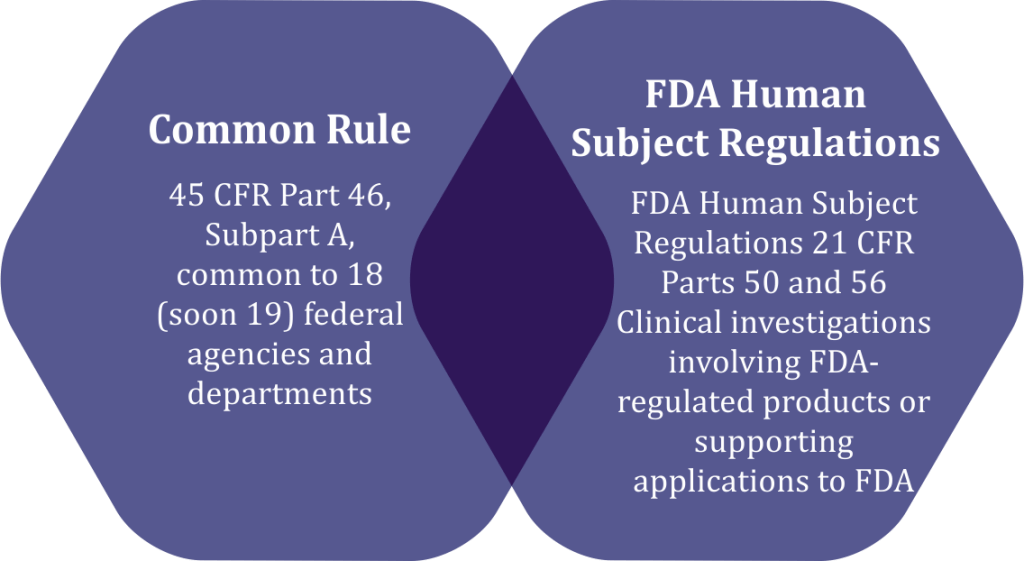
Any research project on human subjects requires clinical trial protocols to be maintained. It is the duty of the Institutional Review Board (IRB) to ensure that all types of research conducted on human subjects is in accordance with federal, state, institutional, and ethical guidelines. In short, the IRB plays an important role in protecting the rights and welfare of human subjects involved in clinical trials.
Regular meetings by the IRB committee are essential to make sure that research institutions are compliant with FDA regulations and following the protocol before and during human trials. IRB meetings help address issues with protocol review and non-compliance, and solve unanticipated problems.
When a meeting is called, it is expected that a majority of the committee members will actively participate and contribute to the review. However, it may so happen that members are unavailable to attend face-to-face meetings. Since skipping these meetings might adversely affect IRB productivity, electronic IRB meetings are being increasingly adopted to facilitate ease of attendance and to implement an easier way to review protocols.
Here are some tips to extract the most benefit out of your IRB compliance software:
- Prepare a list of the important parts of the meeting – agenda, reviews, minutes, etc. are the standard features of a meeting. You can add more elements based on the requirements (statement by Chair, meeting reminder, etc.).
- Include all relevant details in the agenda about the item(s) being discussed and/or reviewed. This will help participants get a better understanding of the meeting agenda.
- Use the electronic system to give reviewers full access to review items and comments. Reviewers should be able to access the notes and checklists.
- Present recommendations for each protocol should be listed. This shows the importance of recommendations and gives members a good idea about each protocol.
- Have a separate and detailed section for the recommendations. Encourage all members to share their experience, provide their inputs and make recommendations.
- Discuss and issue protocol approvals during the meeting. Document the protocol discussions.
- Generate minutes of the meeting using the Minutes Builder tool in the application. If entering minutes in real time is too difficult, minutes can be added later on. For better clarity, changes and/or edits can be made in the minutes with the help of the Edit Minutes function.
- Use letter templates in the software to design follow-up letters for IRB members based on the minutes of the meeting. This summarizes the entire meeting, even though members have not been physically present.
A good quality IRB compliance software for conducting IRB meetings not only eliminates the unproductive elements of a traditional meeting (such as keeping records on paper, physical presence of members and maintaining files) but also facilitates a large number of ideas and recommendations to be generated, thus giving rise to a more unbiased, productive and effective review.