Automate validation to catch missing or incomplete information before review. Reduce errors and ensure protocols reach the IBC committee complete and ready for evaluation.

Automatically route protocols based on risk level and biohazard classification. Smart workflows and automated notifications keep reviews moving without manual follow-ups.

Provide step-by-step guidance on institutional biosafety requirements. Automated prompts flag needed documentation for recombinant DNA, infectious agents, and select agents before submission.

Reference approved IRB and IACUC protocols seamlessly when biohazards are involved. This in-built integration eliminates duplicate entry and maintains version control across amendments.


















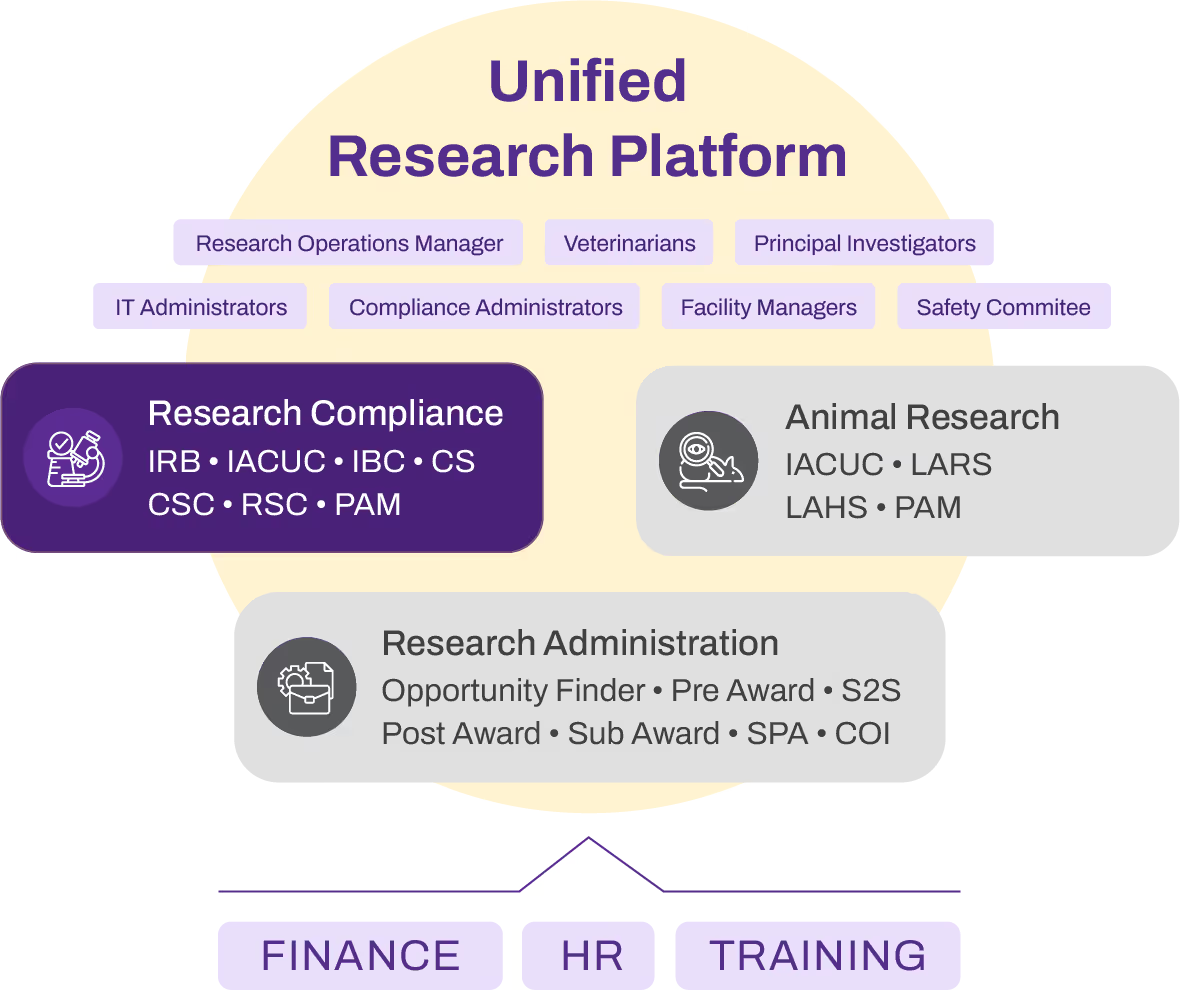

The IBC module integrates seamlessly with IRB and IACUC systems, ensuring a unified compliance ecosystem. This integration allows for smooth data sharing across protocols, so relevant information from IRB and IACUC applications can be automatically referenced, reducing manual data entry and ensuring consistency across all research protocols.

The IBC module provides comprehensive tracking for every protocol, including amendments, renewals, and deviations. A centralized dashboard gives you complete visibility into protocol status, ensuring compliance across all research activities.

By automating protocol documentation, routing, and review processes, the IBC module flags compliance issues early and ensures all protocols are reviewed by the right people at the right time. This reduces errors, accelerates approval times, and ensures consistent, high-quality compliance tracking.

Absolutely! The IBC module is scalable and can be tailored to fit the unique needs of different industries, including universities, biotech, pharma, research institutions, and federal agencies. Whether you’re dealing with recombinant DNA or other biological agents, it adapts to your specific compliance requirements.

The IBC module automatically generates audit-ready documentation, tracks amendments, renewals, and deviations, and maintains a full, chronological record of all protocol actions. This ensures that you are always prepared for inspections and that your biosafety protocols meet federal and institutional compliance standards.

No, the IBC module is designed to be user-friendly and intuitive. We provide easy-to-follow training and support materials so your team can quickly get up to speed and start using the module without requiring extensive technical training.

The IBC module automatically tracks protocol versions, ensuring all stakeholders work from the most current, approved protocol. It also centralizes protocol data, allowing for real-time access and collaboration across departments, eliminating miscommunication and speeding up the review process.