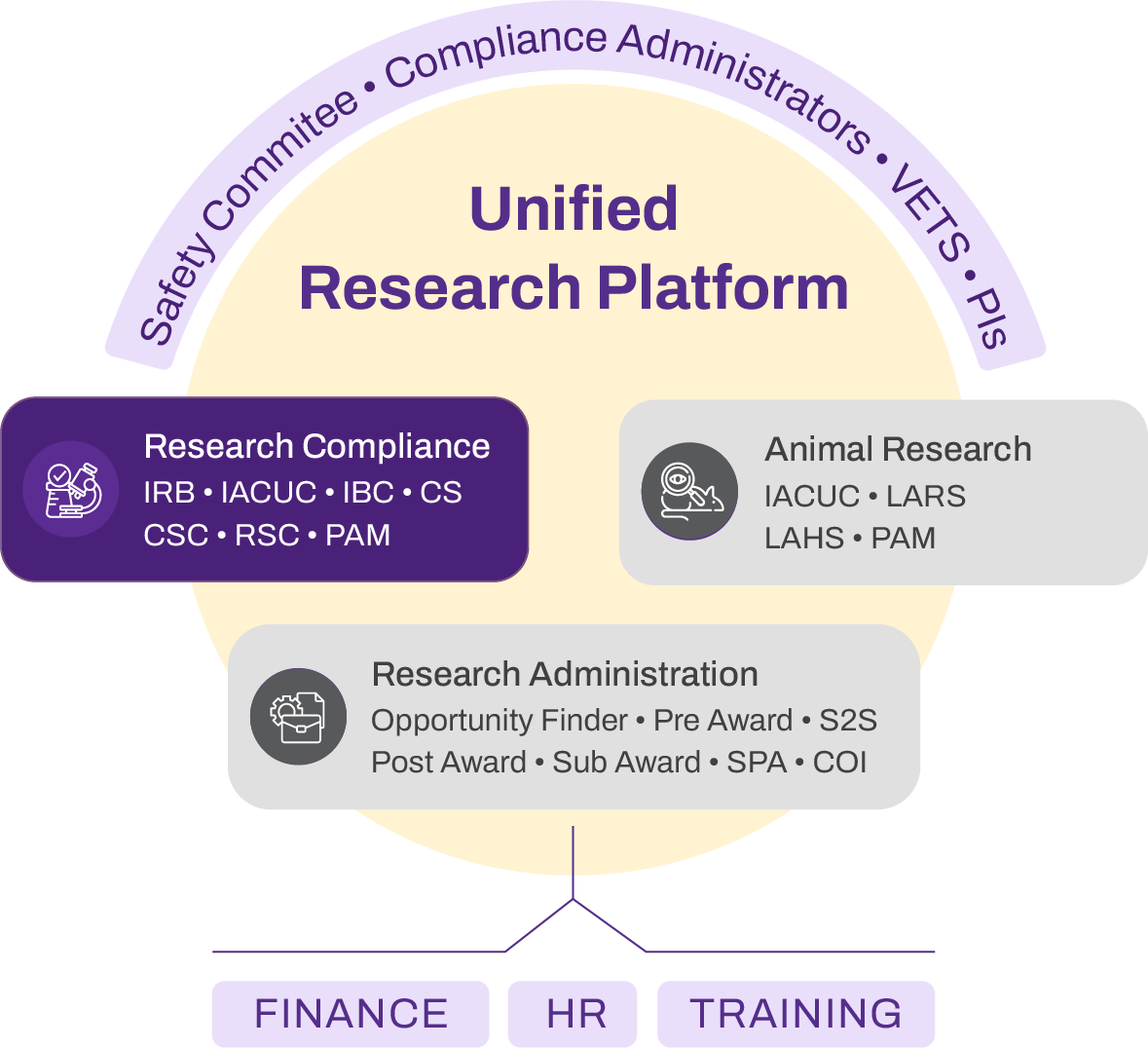
This brochure provides a comprehensive overview of eProtocol for animal research protocol management. Inside, you'll find detailed information on protocol lifecycle workflows, compliance documentation features, and institutional oversight capabilities. Download the PDF to share with your IACUC committee, IT team, or procurement stakeholders.
What you’ll learn in the brochure
Automated protocol processing
See how eProtocol streamlines submissions, routing, and reviews in the detailed workflow diagrams included in the brochure.
Electronic documentation with audit-ready records
Review how the system captures all protocol activity in one place, with traceability that supports oversight and reporting
Integrated review management
Learn how to manage reviews, renewals, amendments, and deviations within a single workflow.
Centralized repository
Explore the technical architecture for maintaining a unified institutional record to support efficient operations and consistent program oversight.

.png)