






Explore our upcoming events and see what’s next in compliance and digital solutions.

The CSC module simplifies protocol management by automating the creation, submission, review, and approval of protocols. It ensures that all required compliance documentation is easily accessible and up to date, helping reduce approval times and administrative delays. The system integrates seamlessly with your existing compliance systems (e.g., IRB, IACUC) to centralize all data, making it easier for stakeholders to access and review protocols in one place.

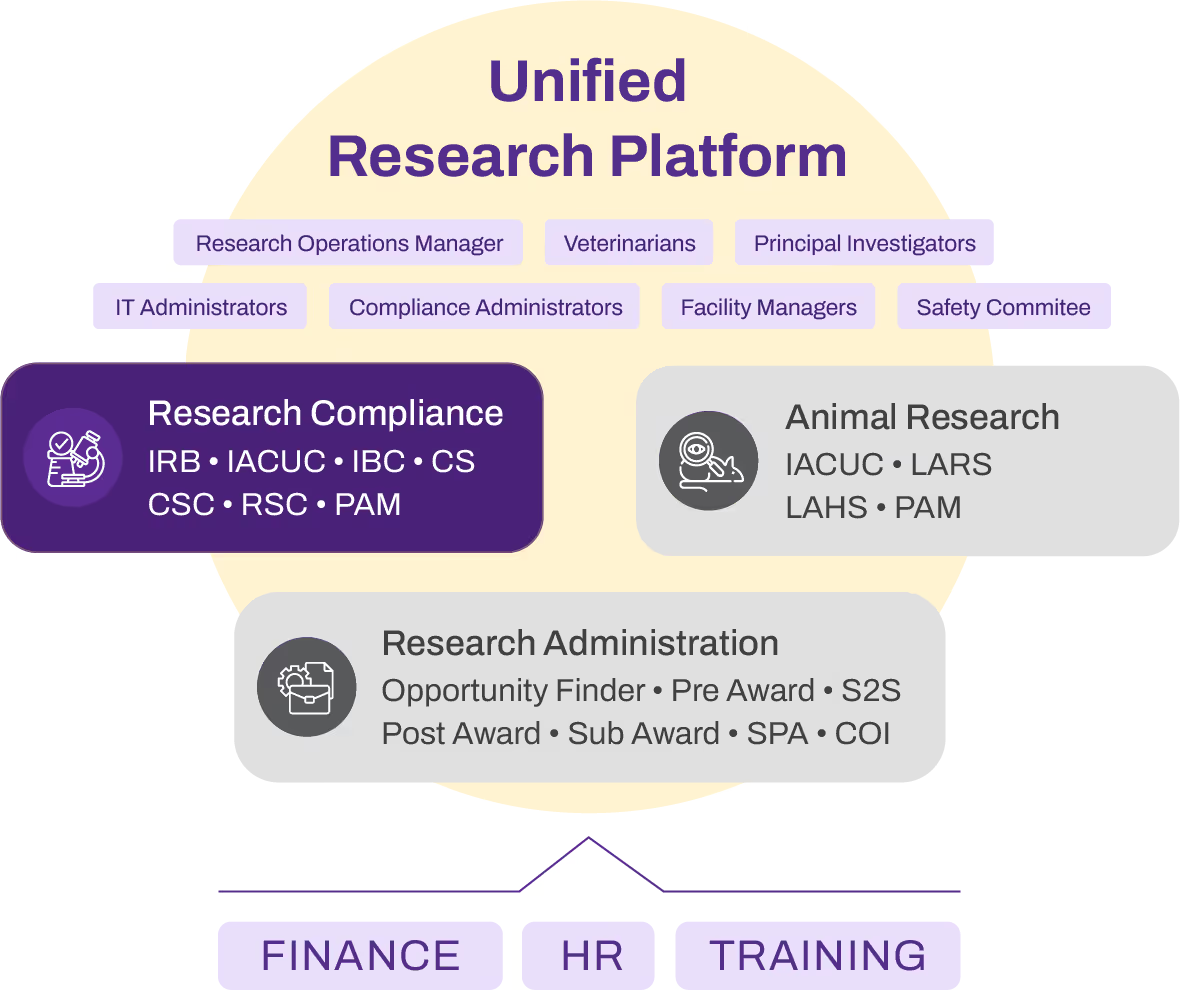
Yes, the CSC module is designed for easy integration with other systems you may already use, such as IRB, IACUC, HR, training records, and finance systems. This ensures that all necessary data for protocol creation, submission, and approvals is readily available and can be accessed in one unified platform, eliminating the need for manual data entry and reducing inefficiencies caused by switching between multiple platforms.

The CSC module ensures compliance by providing a centralized system for managing protocol documentation, including SDS (Safety Data Sheets), hazard assessments, PPE usage, and other safety protocols. It generates comprehensive regulatory reports with just a few clicks, enabling you to stay audit-ready at all times. All protocols, approvals, amendments, and revisions are automatically logged with full audit trails, ensuring all required documentation is available for inspection whenever needed.

The CSC module simplifies the management of protocol amendments, renewals, and adverse events by using smart forms that facilitate easy submission, review, and tracking. The system automatically logs all changes and updates to protocols, ensuring that all revisions are documented and compliant with safety standards. It also streamlines the process of reporting adverse events, ensuring that they are promptly addressed and tracked for compliance.

Yes, the CSC module enables you to generate customizable, comprehensive regulatory reports with ease. Whether it’s EPA submissions, safety audits, or any other required compliance reports, the system automates the process, allowing you to generate reports at the click of a button. This ensures that you meet regulatory requirements without spending excessive time on manual report generation.

The CSC module automates the routing of protocols to the correct stakeholders for review and approval, significantly reducing approval times and eliminating bottlenecks. It also provides a platform for electronic reviews, where meeting agendas, minutes, and decisions are recorded and tracked in real time. This paperless process ensures that all protocol changes, amendments, and approvals are efficiently managed and documented.

The CSC module maintains complete audit trails for all protocol management activities, including submissions, reviews, approvals, amendments, and any changes made to protocols. Every action is recorded and time-stamped, ensuring transparency and accountability. These audit trails make it easy to demonstrate compliance during regulatory inspections or internal audits.